
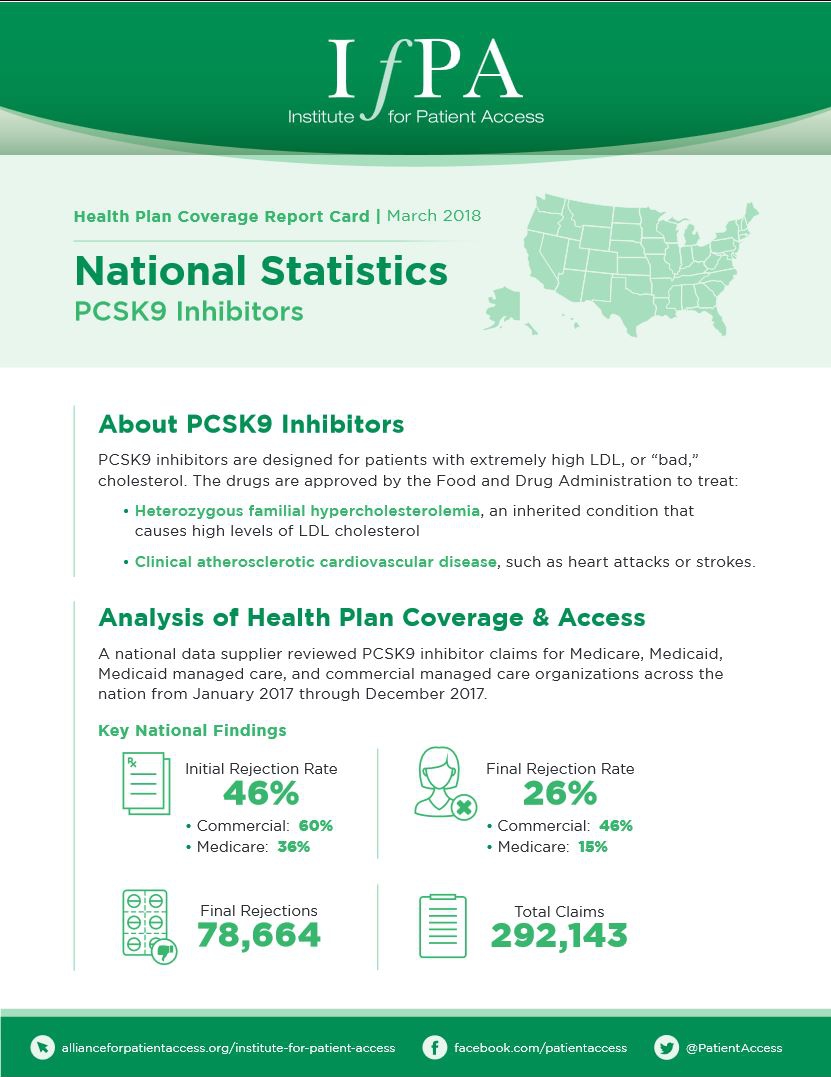
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for Medicare, Medicaid, Medicaid managed care, and commercial managed care organizations across…
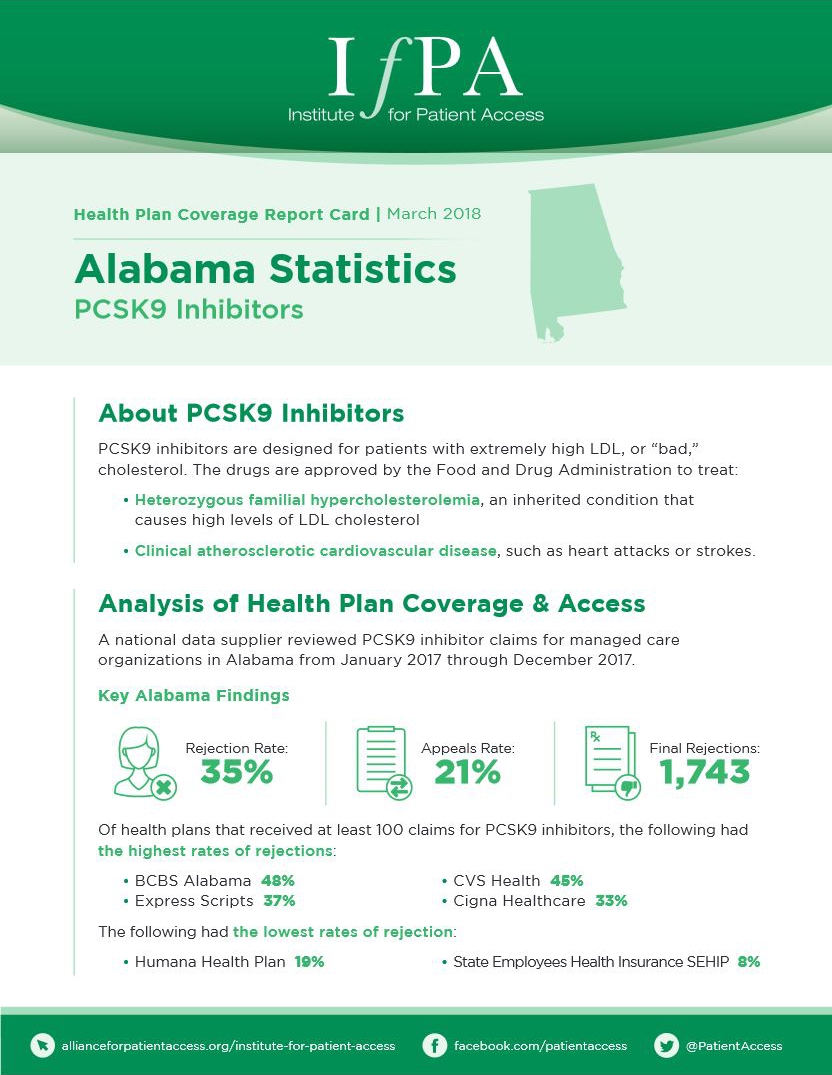
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in Alabama from January 2017 through December 2017.…

March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in California from January 2017 through December 2017.…
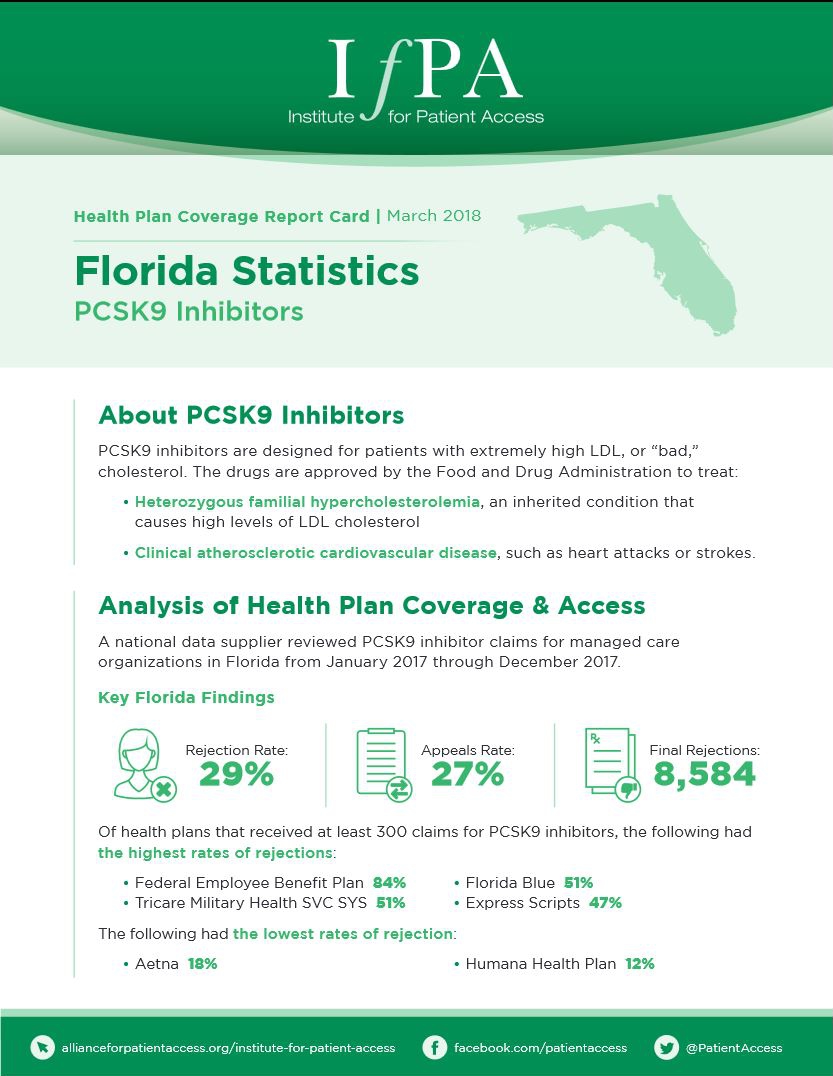
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in Florida from January 2017 through December 2017.…
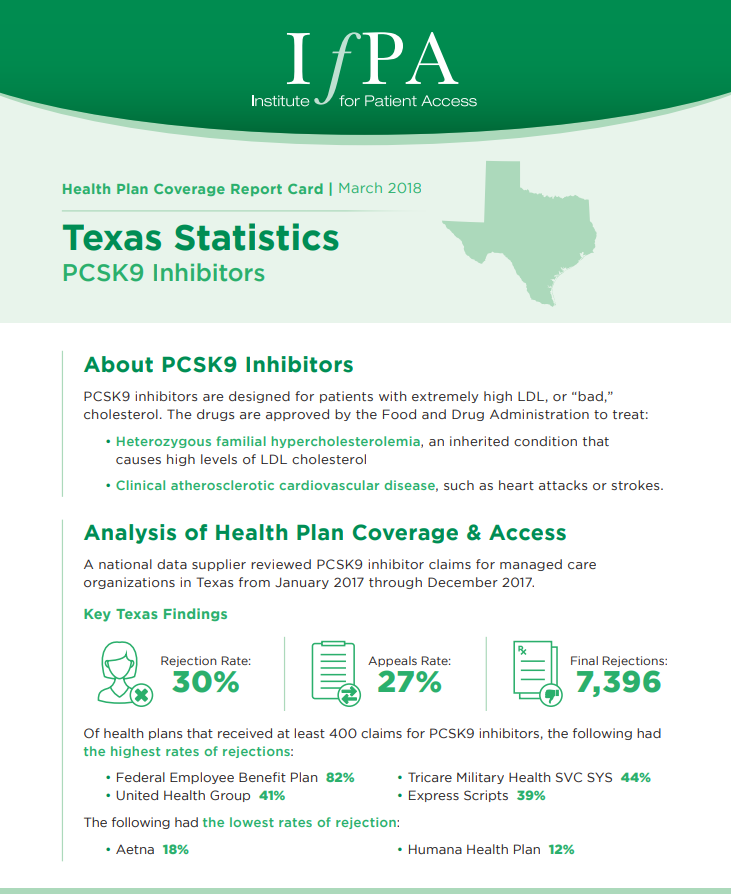
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in Texas from January 2017 through December 2017.…
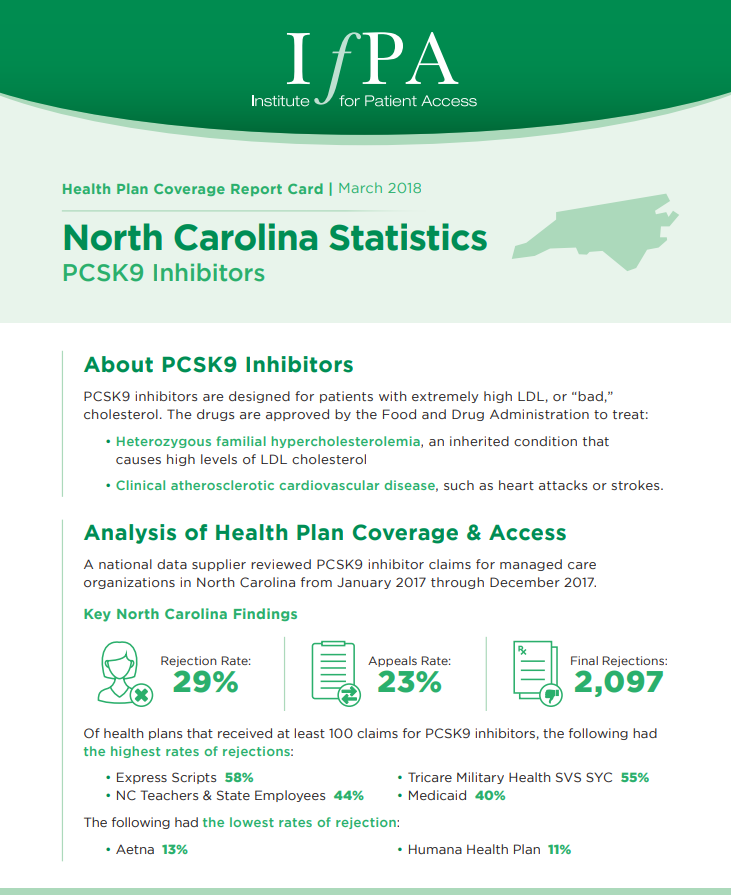
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in North Carolina from January 2017 through December…

March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in New York from January 2017 through December…
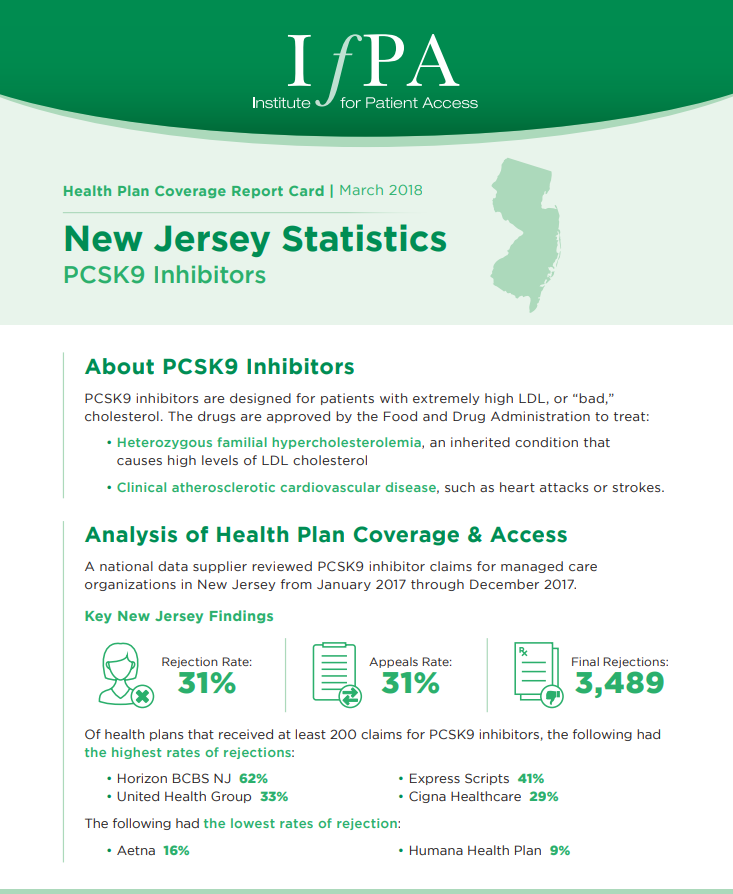
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in New Jersey from January 2017 through December…
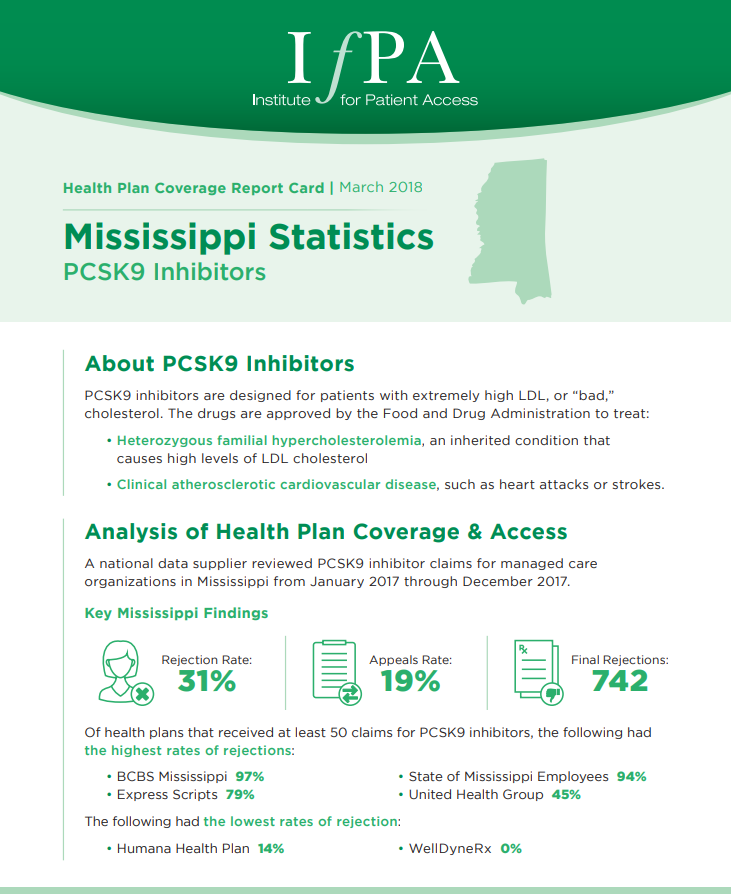
March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in Mississippi from January 2017 through December 2017.…

March 1, 2018
A national data supplier reviewed PCSK9 inhibitor claims for managed care organizations in Indiana from January 2017 through December 2017.…












