

October 20, 2016
This month’s World Health Organization Open Sessions with INN Stakeholders explored the value, and the future, of the biological qualifier system. The Global Alliance for Patient Access was pleased to present to WHO officials and an international group of fellow stakeholders.

October 13, 2016
Biosimilar medicines are being prescribed more and more freely across Europe. But access to, and use of, these new medicines differs greatly depending on which European country you live in.
October 6, 2016
Since the advent of biosimilars, experts and pundits have debated how reduced biosimilar prices will be and just much savings the follow-on drugs will offer.
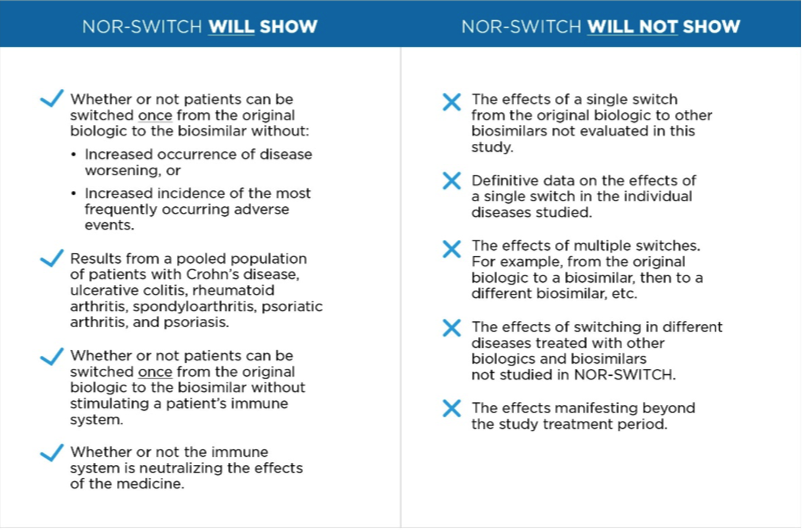
September 15, 2016
Data from a clinical study in Norway may soon explain the effects of switching stable patients from a biologic medicine, infliximab, to its biosimilar counterpart. But, as the Global Alliance for Patient Access argues in a new white paper, policymakers must accurately interpret what the NOR-SWITCH study will – and will not – demonstrate about the safety of switching.
August 9, 2016
Biosimilar medications designed to give patients more options may instead become health plans’ newest tool for limiting patient access.
July 20, 2016
July 12 and 13 hearings of the FDA’s Arthritis Advisory Committee cleared the way for two new biosimilars, adalimumab and etanercept. The committee unanimously supported manufacturers’ applications for the drugs – but that wasn’t the only point of consensus. Participants and even committee members expressed concerns about unfinished regulatory policy and the need to prioritize patient safety.
July 12, 2016
Breathing complications aren’t the only challenge faced by the 26 million Americans with asthma, explains a new video from the Alliance for Patient Access.
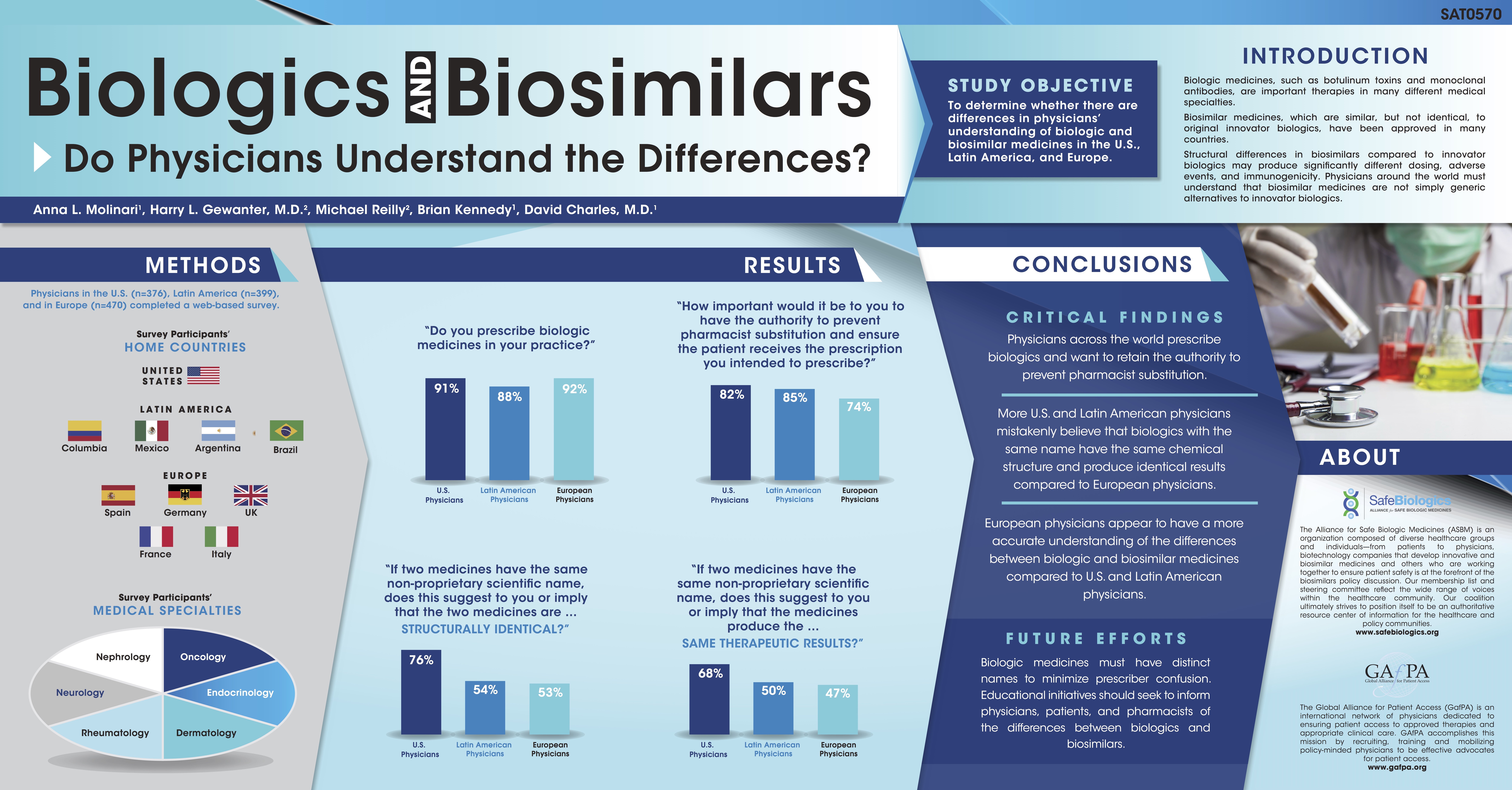
July 5, 2016
Patient advocates have teamed up to take an important message to European physicians and policymakers: distinct names for biosimilar medications are essential.
June 21, 2016
Stakeholders are divided over a provision of the Food and Drug Administration’s draft regulatory guidance on biosimilar labeling, letters to the FDA reveal.

June 15, 2016
As more biosimilars become available globally, clinicians are eager to understand: How does switching between biological medicines affect patients?








