
August 2, 2016
Congress returns in September from a seven-week recess—and ready, many patients and advocates hope, to push the 21st Century Cures Act over the finish line. Senator Lamar Alexander (R-Tenn.) expects the Senate to resume work on the bill, which he says could be “the most important legislation Congress passes this year.” The bill passed the House of Representatives in July 2015 with bipartisan support.
July 20, 2016
July 12 and 13 hearings of the FDA’s Arthritis Advisory Committee cleared the way for two new biosimilars, adalimumab and etanercept. The committee unanimously supported manufacturers’ applications for the drugs – but that wasn’t the only point of consensus. Participants and even committee members expressed concerns about unfinished regulatory policy and the need to prioritize patient safety.
July 7, 2016
The Centers for Medicare and Medicaid Services’ Oncology Care Model is off and running. As announced during The White House’s cancer summit at Howard University, more than 200 physician practices and 3,200 oncologists have enrolled – effective July 1.
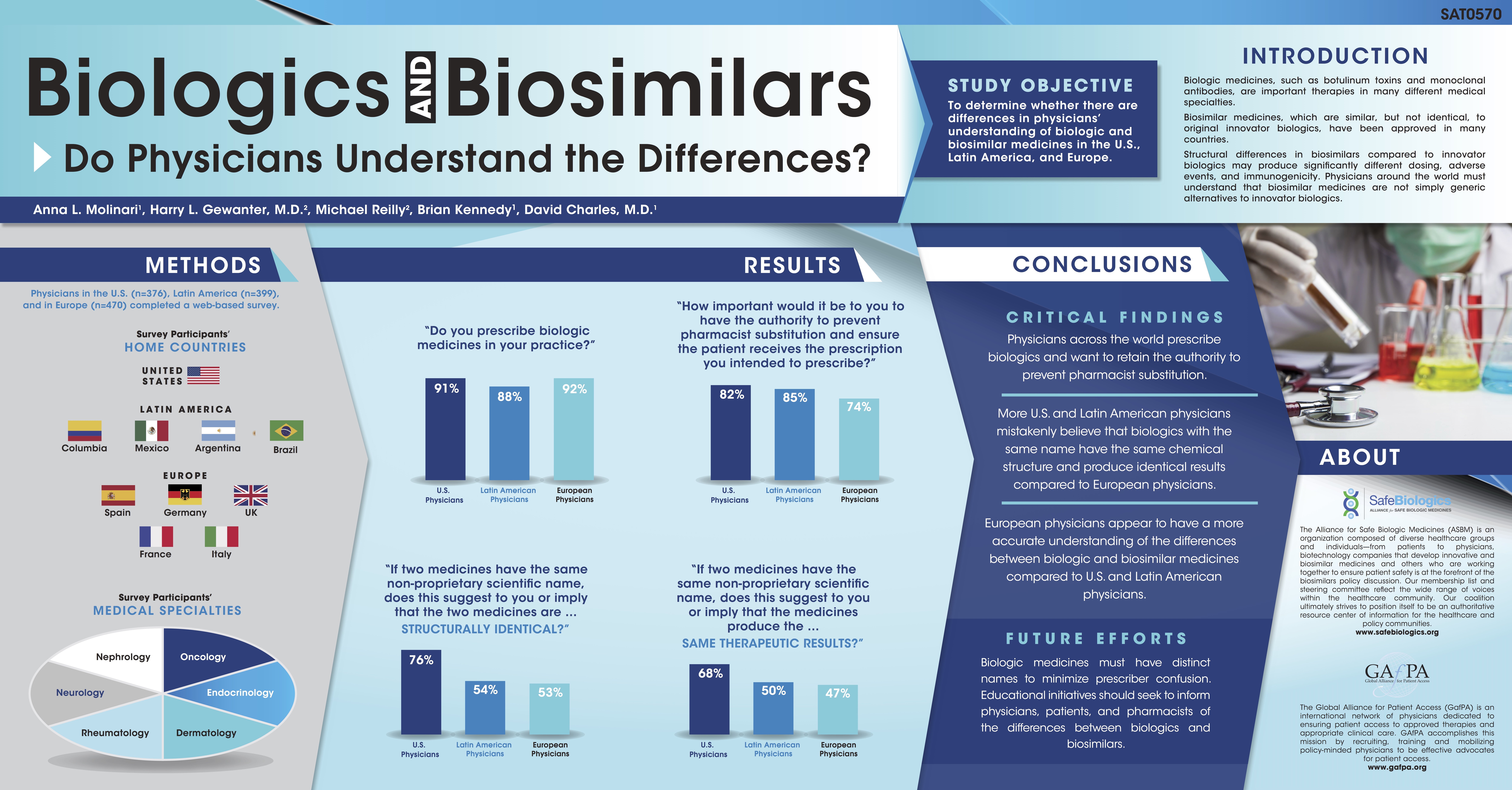
July 5, 2016
Patient advocates have teamed up to take an important message to European physicians and policymakers: distinct names for biosimilar medications are essential.
June 30, 2016
Starting July 1, patients battling hepatitis C in Delaware will have newfound access to direct-acting antiviral cures – a victory now shared with patients in New York, Pennsylvania, Florida and Washington.
June 28, 2016
The opioid abuse epidemic continues to generate state laws aimed at reducing opioid misuse and overdose.
June 23, 2016
High drug prices have led to a proliferation of value models that investigate how the United States can control ballooning health care costs. But just how realistic are these models – and their implementation?
June 21, 2016
Stakeholders are divided over a provision of the Food and Drug Administration’s draft regulatory guidance on biosimilar labeling, letters to the FDA reveal.
June 9, 2016
Health care in 2017 is shaping up to be expensive. Citing financial losses, private insurers who offer health plans through an Affordable Care Act exchange want to increase their premiums – some by double digits.
June 7, 2016
Vice President Joe Biden addressed oncologists, industry and medical professionals in Chicago Monday with a resounding plea: collaborate to “translate scientific discovery into meaningful treatments.”


