40% of Babies Denied Preventive Treatment by Insurers
April 21, 2020
Parents of premature babies face enough challenges. Trying to access medication that could keep their baby safe from respiratory syncytial virus, a potentially deadly disease, shouldn’t be one of them. But new national data shows that’s the case.
A recently released Institute for Patient Access report card examines insurance claims for palivizumab, the preventive treatment that protects premature infants from RSV. The report card summarizes claims from January through December 2019, including data from both commercial plans and Medicaid.
Key Findings
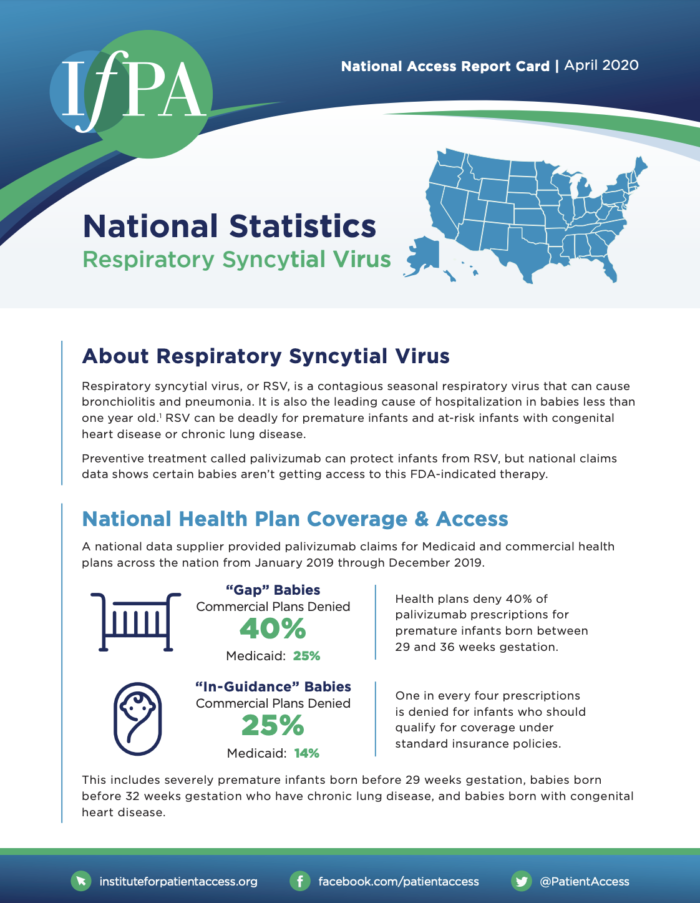
“Gap” Babies
Premature infants born between 29 and 36 weeks gestation are subject to high rates of denial.
- 40% denied by commercial plans
- 25% denied by Medicaid
These infants are sometimes called “gap” babies because they fall into an insurance coverage gap. They have a higher gestational age than severely premature infants, who are generally covered by insurance policies, and a lower gestational age than term babies, who may not need palivizumab.
Health plans regularly deny coverage for preventive RSV treatment for infants born 29-36 weeks gestation based on 2014 clinical guidelines suggesting that only severely premature infants needed protection. This recommendation has the effect of keeping babies from getting the preventive treatment their health care providers have prescribed.
“In-Guidance” Babies
Even one in four high-risk babies typically covered by insurance policies is going without the preventive RSV treatment their health care provider prescribed.
- 25% denied by commercial plans
- 14% denied by Medicaid
These “in-guidance” babies are severely premature babies born before 29 weeks gestation, babies born before 32 weeks gestation with chronic lung disease, and babies born with congenital heart disease.
Reactions
The rejection of prescriptions for at risk infants concerns infant health providers. Just ask National Coalition for Infant Health Medical Director Mitchell Goldstein, MD.
“Now more than ever, we’re aware of the importance of preventing infectious disease,” Dr. Goldstein said. “Those faced with the challenge of bringing home a preemie or at-risk infant have enough on their mind. They shouldn’t have to fight their insurance company for a medication that can protect their baby from complications of infectious disease.”
Suzanne Staebler, DNP, noted that the issue is not a new one. “This data confirms what we’ve been seeing for years,” Staebler explained, “that misguided policies are putting fragile babies at unnecessary risk. Clinicians prescribe this preventive treatment to vulnerable infants because they need it.”
Preventing RSV
While most children get RSV before the age of two, the virus can be deadly for premature infants with underdeveloped lungs and immature immune systems. RSV is the leading cause of hospitalization for children under age one.
Palivizumab is FDA approved for all premature infants, all infants with congenital heart disease and infants born before 32 weeks with chronic lung disease. The preventive medication reduces RSV infections and decreases hospitalizations by 55%.
Categorized in: Blog